Category: Tech highlight
Beyond Flow Cytometry: Unlocking Single-Cell Insights with 10x Genomics
Bridging Flow Cytometry with Single-Cell Sequencing
Flow cytometry has long been the backbone of high-dimensional single-cell analysis. With the ability to measure up to ~45 parameters per cell, flow has enabled researchers and core facilities to rapidly phenotype complex populations and enrich rare cell types with confidence.
But what if you could go further — without disrupting your existing workflows?
By integrating flow cytometry with single-cell sequencing, researchers can now move beyond predefined panels and surface markers to uncover deeper, unbiased biological insight.
Why Bridge Flow Cytometry with Single-Cell Sequencing?
Flow cytometry has long been the gold standard for measuring what you expect to see. But what about the biology you don’t expect? What about the rare cell states, subtle activation programs, and novel biomarkers that surface markers alone simply can’t capture?
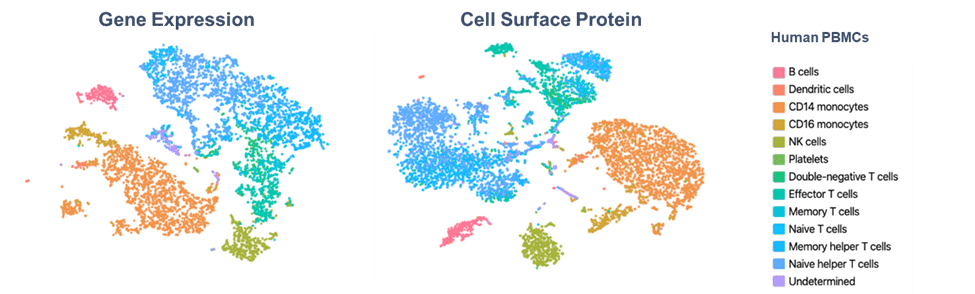
This is where single-cell sequencing transforms your capabilities. By bridging flow cytometry with the 10x Genomics platform, you can move from measuring dozens of parameters to profiling 300+ protein markers—with the option to simultaneously capture whole-transcriptome gene expression from the same single cell.
Unmatched multiplexing without compromise
Breaking free from spectral overlap limitations opens new possibilities. Instead of carefully balancing fluorophore combinations, you can now interrogate hundreds of protein markers alongside comprehensive gene expression data. This quantum leap in multiplexing reveals cellular complexity that traditional flow simply cannot access.
Discovering What’s Hidden
Many critical cell states appear identical by surface protein analysis alone. Activation, exhaustion, differentiation, and stress states often require deeper molecular investigation to truly distinguish. Single-cell sequencing uncovers these hidden layers of heterogeneity, revealing novel populations and functional states that would otherwise remain invisible.
Deeper biology from enriched populations
Leverage FACS to isolate rare or complex populations, then apply single-cell sequencing to achieve transcriptome-wide and high-plex proteomic resolution.In short: sort with flow, then profile with depth.
Designed for Your Workflow
The beauty of this approach is that it builds on what you already do exceptionally well. Use FACS to enrich the rare or complex populations you’re interested in, then apply single-cell sequencing to achieve transcriptome-wide resolution. Your expertise in flow cytometry becomes the foundation for even more powerful discoveries.
What Can You Unlock?
By pairing flow cytometry with single-cell sequencing, researchers can access a new layer of biological resolution:
-
Unbiased cell type and state discovery
Identify known and novel populations without relying on predefined antibody panels. -
Protein validation with gene expression
Confirm antibody signals and distinguish protein presence from true pathway activation. -
Functional state resolution beyond surface markers
Dissect activation, exhaustion, differentiation and stress states that appear identical by flow. -
Pathway-level biology at scale
Interrogate canonical pathways such as Wnt, TCR and interferon signalling through coordinated gene expression, rather than single proxy markers.
New Flexible and Cost-Effective Options
With the introduction of Single Cell Flex v2.0 (Protein-Only), high-plex single-cell protein profiling is now more accessible than ever.
This flexible option enables:
-
High-plex protein analysis at a significantly reduced cost
-
Support for more projects, more users and more samples
-
No requirement for RNA profiling, where transcriptomics isn’t needed
For many flow cores, this opens the door to offering advanced single-cell services without the overhead of full transcriptome assays.
The Natural Next Step for Flow Cytometry
The integration of 10x Genomics technology with your existing flow cytometry services doesn’t mean disrupting established workflows—it means enhancing them. Your team’s expertise in high-dimensional single-cell analysis positions you perfectly to take this next step.
As the authorised 10x Genomics distributor in Australia and New Zealand, Millennium Science is here to help you understand how single-cell sequencing can complement and extend your current offerings. Whether you’re looking to add new capabilities, support cutting-edge research, or simply explore what’s possible beyond traditional flow, our specialists are ready to guide you through the options.
Ready to expand your core’s capabilities?
Contact our team today to learn more about 10x Genomics solutions and how they can seamlessly integrate into your core facility workflows.
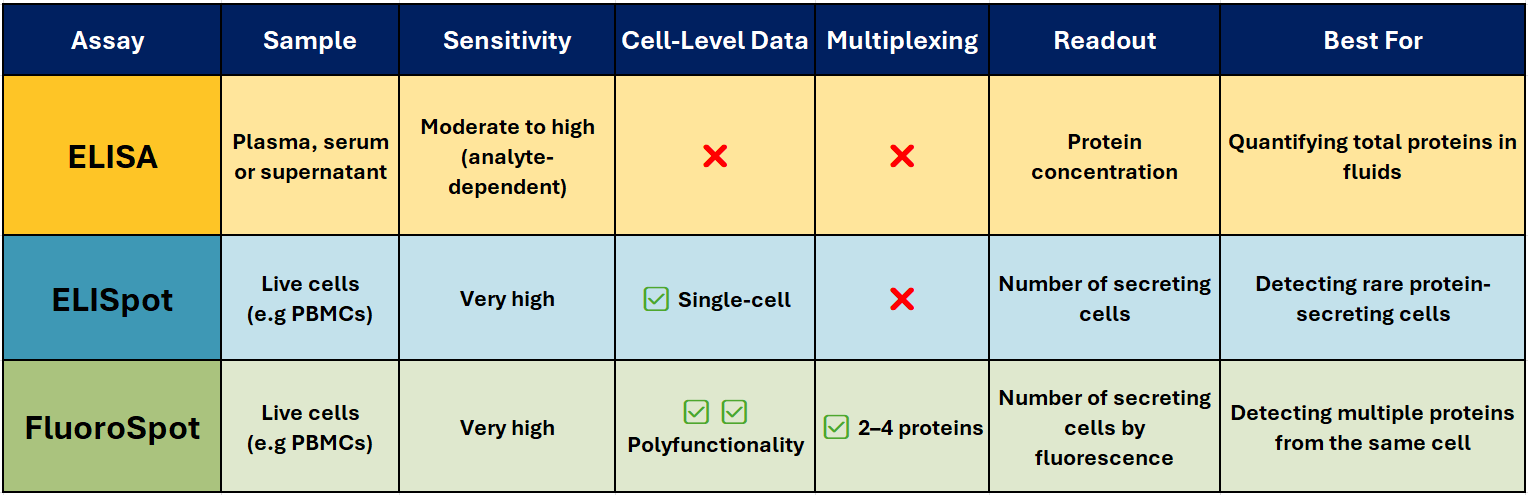
Spot the Difference: ELISA vs ELISpot vs FluoroSpot
A Joe Blogs post by Joe Roberts, PhD
When it comes to measuring proteins such as cytokines, antibodies, or growth factors, there’s no shortage of options. ELISA, ELISpot, and FluoroSpot all have important roles to play. While each assay relies on antibody-based detection, knowing which one to use (and when) can mean the difference between simply collecting data and gaining real insight.
As a Product Manager at Millennium Science, I speak with researchers every day who are deciding between these techniques. In this blog we will explore each assay to help you spot the difference and help you choose the right assay for your next experiment.
ELISA – The Trusted Workhorse 📊
Best for: Quantifying total soluble protein in biological fluids.
ELISA (Enzyme-Linked Immunosorbent Assay) is one of the most widely used immunoassays for detecting and quantifying soluble proteins, such as cytokines, antibodies, and hormones, in serum, plasma, or cell culture supernatants. It’s robust, scalable, and ideal for high-throughput analysis when you need accurate, reproducible concentration data.
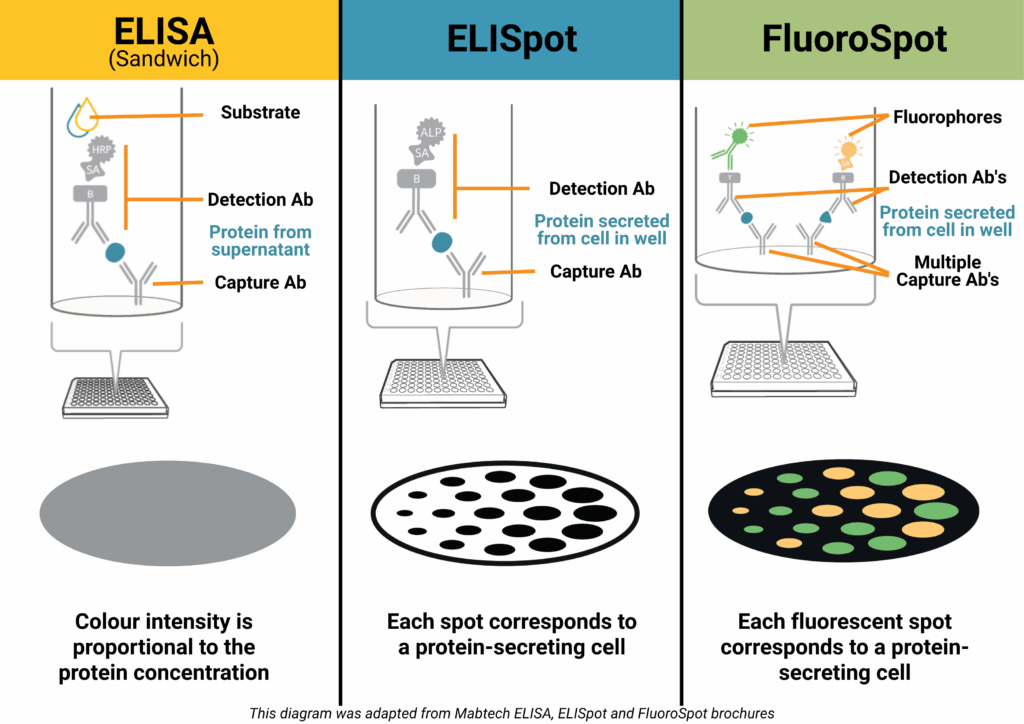
How it works (Sandwich ELISA):
A capture antibody is coated onto a high-binding plate and binds the target protein in your sample. A biotinylated detection antibody binds a different epitope, followed by a streptavidin–enzyme conjugate. Addition of a colourimetric substrate produces a measurable signal proportional to protein concentration.
Why researchers choose it:
- Delivers quantitative results (e.g., pg/ml or ng/ml)
- Compatible with high-throughput and automation
- Well-established, with widely available equipment
- Ideal for comparative analysis across multiple samples
Limitations:
- No information on the number or type of cells producing the protein
- Sensitivity can be lower than single-cell assays
ELISpot – Spotlight on Secreting cells
Best for: Counting individual protein-secreting cells.
ELISpot (Enzyme-Linked ImmunoSpot) detects and counts cells that secrete a specific protein. It’s particularly valuable when studying immune responses where the frequency of antigen-specific T or B cells is low – for example in vaccine research, oncology, and autoimmune disease studies.
How it works:
Live immune cells are added to a PVDF plate pre-coated with a capture antibody. When stimulated, the cells secrete the target protein, which is immediately bound near the cell. After washing away cells, a detection antibody and enzyme conjugate are added. A precipitating substrate forms a visible spot at each secretion site – each spot representing a single responding cell.
Why it stands out:
- Extremely sensitive – can detect one responder cell in >100,000
- Functional readout of immune activity
- Ideal for rare antigen-specific responses
- Widely used in T-cell response monitoring, vaccine trials, allergy research, and immuno-oncology
Pairing with Mabtech’s ASTOR2 automated ELISpot reader delivers rapid, high-precision spot counting with consistent, reproducible results across entire assay plates, and when used with Mabtech monoclonal antibody pairs and ready-to-use ELISpot kits, ensures reliable performance across species and sample types.
FluoroSpot – Multiplex Made Easy 🌈
Best for: Analysing polyfunctional immune responses.
FluoroSpot builds on the ELISpot principle but uses fluorescently labelled detection antibodies to identify multiple proteins secreted by the same cell – typically 2-4 proteins. This allows you to measure not just whether a cell responds, but how it responds.
Why it’s powerful:
- Detects polyfunctionality – cells secreting multiple cytokines
- High sensitivity with low background
- Enables simultaneous detection of different functional subsets
- Excellent for vaccine research, infectious disease, and cancer immunotherapy
Pairing with Mabtech’s IRIS2 ELISpot, FluoroSpot and FociSpot reader enables precise, high-resolution spot detection across multiple fluorescence channels.
Joe’s Takeaway
- ELISA → When you want to know how much protein is present in a sample.
- ELISpot → When you need to know how many cells are producing a protein.
- FluoroSpot → When you need to know which protein a cell is producing, and whether it’s producing more than one at the same time.
In many projects, using ELISA and ELISpot/FluoroSpot together can give the clearest picture — combining quantitative bulk measurements with functional single-cell insights.
If you’d like help selecting the right assay for your research, get in touch with our team at Millennium Science. We’re local experts, and Mabtech’s range covers everything from research use to clinical-grade applications.
Until next time… happy experimenting!
Joe Roberts, PhD
Product Manager
Millennium Science